

TREATMENTS
Exploring new frontiers for the treatment of Musculoskeletal Disorders

TREATMENTS
Exploring new frontiers for the treatment of Musculoskeletal Disorders
17+ Years of using patented and proven stem cell treatment for Musculoskeletal Disorders
Musculoskeletal Disorders (MSD) are a group of genetic diseases that cause progressive weakness and loss of muscle mass. In muscular dystrophies, abnormal genes (mutations) lead to muscle degeneration. Most forms begin in childhood. There are different forms of MSD that vary in symptoms, problems and severities. This cluster of genetically diverse disorders share clinical and pathological characteristics but differ in the age of onset, the rate of progression, distribution of weakness, severity, inheritance pattern and molecular defect. Patients become progressively weaker. Most people who have the condition eventually need a wheelchair. Other symptoms include trouble breathing or swallowing.

Traditionally the treatment for MSD involves multiple disciplines like pediatric neurology, pediatric orthopaedic surgery, pediatric critical care, physical medicine &rehabilitation, physical training, occupational therapy, behavioural guidance, etc. Medication, therapy, breathing aids or surgery may help maintain function and manage pain, not a complete reversal of damage due to disease. With Stem Cell Treatment, the activated stem cells can reach the targeted site to form new muscle fibres or fuse with the existing cells to repair the damage.

Stem cells are administered in the body through veins or lumbar puncture. Once infused into the muscles at the targeted site they can be tempted to be muscle fibre cells and also can reduce the inflammation; thereby slowing the progression of the disease. Stem cells differentiate into the high purity motor neuron progenitor cells that have the ability to regenerate new and healthy neurons helping with the condition. After successful therapy, patients have shown remarkable improvement such as increased trunk muscle strength, limb strength on manual muscle testing and balance.

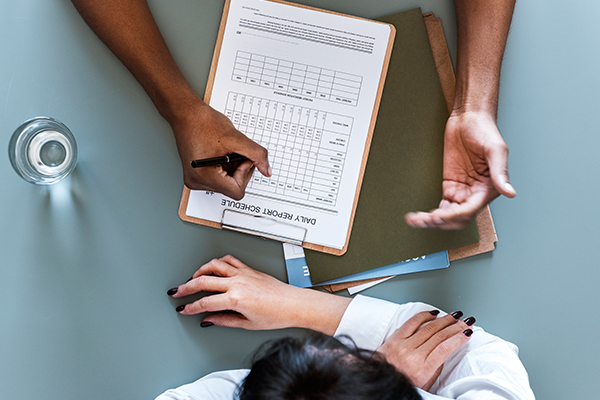
At Nutech MediWorld, the patients’ clinical condition is assessed for safety and efficacy of the treatment using conventional systems to check their eligibility for stem cell treatment for Musculoskeletal disorders.

We also conduct an additional round of thorough assessment using our patented NFS (Nutech Functional System) protocols whereby condition-based sets of parameters have been pre-devised for a finer assessment of the patient’s condition (and progress).
After evaluating the specific patient’s condition, a unique/bespoke treatment protocol is decided. This protocol is based on our proprietary stem cell platform, Reostem ®.

The patient’s progress is monitored on a weekly basis to further optimise treatment protocols depending on the patient’s response.

At the end of the treatment, a complete assessment is done to track progress from when the patient came into where he/she has reached now. If required, a follow-up protocol is recommended.

At Nutech MediWorld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Musculoskeletal Disorder patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient.

At Nutech MediWorld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Musculoskeletal Disorders Patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient

At Nutech MediWorld, the patients’ clinical condition is assessed for safety and efficacy of the treatment using conventional systems to check their eligibility for stem cell treatment for Musculoskeletal disorders.
We also conduct an additional round of thorough assessment using our patented NFS (Nutech Functional System) protocols whereby condition-based sets of parameters have been pre-devised for a finer assessment of the patient’s condition (and progress).

After evaluating the specific patient’s condition, a unique/bespoke treatment protocol is decided. This protocol is based on our proprietary stem cell platform, Reostem ®.

The patient’s progress is monitored on a weekly basis to further optimize treatment protocols depending on the patient’s response.

At the end of the treatment, a complete assessment is done to track progress from when the patient came into where he/she has reached now. If required, a follow-up protocol is recommended
We use SCT-N® and SCT-NX® products, based on a proprietary stem cell platform, ReoStem®. ReoStem® is a unique, replicable, clinical-stage technology platform for the generation of allogenic stem cells.
Nutech MediWorld’s regenerative medicine products are universally applicable: they do not require tissue-matching or the co-administration of immunosuppressive drugs.
There is absolutely no evidence of teratoma generation or tumorigenesis associated with SCT-N® or SCT-NX® use in over 17 years of clinical practice across thousands of patients.
Unique cell culture methodology allows scalability of cell expansion and production.