

TREATMENTS
Exploring new frontiers for the treatment of Cerebral Palsy

TREATMENTS
Exploring new frontiers for the treatment of Cerebral Palsy
17+ Years of using patented and proven stem cell treatments for Cerebral Palsy
Cerebral Palsy is a set of neurological conditions caused by hypoperfusion of the brain at or around birth. It can affect muscle movement, coordination and can also cause vision, hearing and cognitive impairments. Types of Cerebral Palsy include Spastic Cerebral Palsy, Dyskinetic Cerebral Palsy, Ataxic Cerebral Palsy & Mixed Cerebral Palsy.

Conventionally, Cerebral Palsy patients are offered rehabilitative therapies like physical therapy, occupational therapy, speech therapy, etc. While these can help manage the symptoms to some extent, they do not address the root cause of Cerebral Palsy ie. damaged brain tissue. Regenerative therapy using stem cells is increasingly becoming the preferred treatment modality for Cerebral Palsy patients.

Stem cell treatment has the potential to repair and regenerate the damaged and non-functional brain cells in Cerebral Palsy patients. It has shown benefits like enhanced motor skills, improved speech and cognitive functions in such patients.

At Nutech Mediworld, we assess the patient’s clinical condition using conventional tools like the GMFCS, MRI etc. to check their eligibility for stem cell treatment for Cerebral Palsy.

We also conduct an additional round of thorough assessment using our proprietary NFS (Nutech Functional System) protocols wherein condition-based parameters are used for a finer assessment of the patient’s condition (and progress).
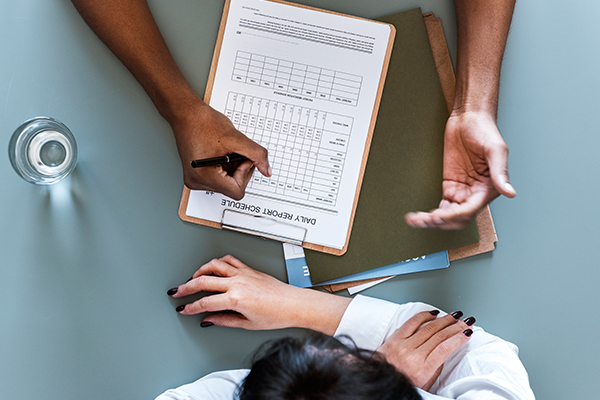
After evaluating the patient’s condition, a customized treatment protocol is created for the patient. This protocol is based on our proprietary stem cell platform, ReoStem®.

The patient’s progress is monitored on a weekly basis to further optimize treatment protocols depending on the patient’s response.

At the end of the treatment period, a complete assessment is done to track progress from when the patient came into where he/she has reached. If required, a follow-up treatment protocol is recommended.

At Nutech MediWorld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Cerebral Palsy patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient.

At Nutech Mediworld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Cerebral Palsy patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient

At Nutech Mediworld, we assess the patients’ clinical condition using conventional tools like the GMFCS, MRI etc. to check their eligibility for stem cell treatment for Cerebral Palsy

We also conduct an additional round of thorough assessment using our proprietary NFS (Nutech Functional System) protocols wherein condition-based parameters are used for a finer assessment of the patient’s condition (and progress)

After evaluating the patient’s condition, a customized treatment protocol is created for the patient. This protocol is based on our proprietary stem cell platform, ReoStem

The patient’s progress is monitored on a weekly basis to further optimize treatment protocols depending on the patient’s response

At the end of the treatment period, a complete assessment is done to track progress from when the patient came into where he/she has reached. If required, a follow-up treatment protocol is recommended
We use SCT-N® and SCT-NX® products, based on a proprietary stem cell platform, ReoStem®, which comprises of a unique, replicable, clinical-stage technology for the generation and clinical application of allogenic stem cells.
Nutech Mediworld’s regenerative medicine products are universally applicable: they do not require tissue-matching or the co-administration of immunosuppressive drugs.
There is no evidence of teratoma generation or tumorigenesis associated with SCT-N® or SCT-NX® use in over 17 years of clinical practice across thousands of patients.
The unique cell culture methodology allows scalability of cell expansion and production.