

TREATMENTS
Exploring new frontiers for the treatment of Brain Disorders

TREATMENTS
Exploring new frontiers for the treatment of Brain Disorders
17+ Years of using patented and proven stem cell treatment for Brain Disorders
Stroke is considered to be a life-threatening incident in which there is an attack on the brain due to blockages in the blood vessels that deprives the brain of sufficient oxygen. A stroke is broadly classified as Ischemic, Haemorrhagic and Transient Ischemic. Ischemic Stroke occurs when the vessel supplying the blood to the different parts of the brain is blocked by the clot. Transient Ischemic Stroke occurs when the supply of blood is blocked by a clot for a relatively short duration of time. Hemorrhagic stroke is resultant of breakage in the blood vessels, interrupting blood flow to an area of the brain.

Conventional treatment for stroke is based on the type of stroke and its severity. The treatment options range from antiplatelet and anticoagulants medications, to surgical procedures like clipping and coiling. Stem cells treatment involves administration of concentrated cells in the targeted area, wherein they can colonize in the damaged area, adapt the properties of resident stem cells and initiate some of the lost functions that have been compromised by the disease or injury.

The Stem Cells are either infused in the cerebrospinal fluid through the subarachnoid spaces of the spinal canal or through the veins to expand blood volumes in the central nervous system, to ensure that the maximum number of cells reach to the targeted area. The vasculogenetic and angiogenetic properties may lead to the formation and development of blood vessels to maximize the supply of the blood to the brain. Stem Cell Treatment at Nutech MediWorld helps reduce obstruction and repair blood vessels to restore and improve control and motor functions in patients with stroke.

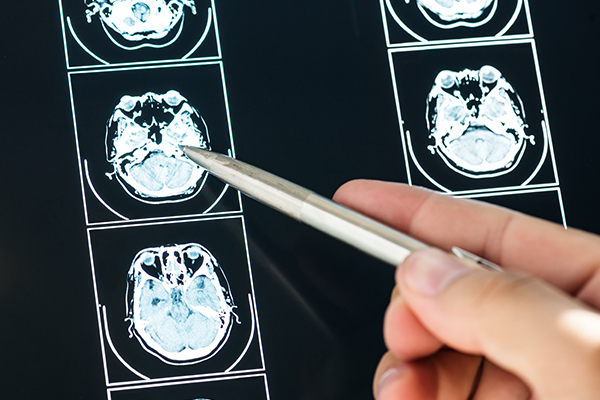
At Nutech MediWorld, the patients’ clinical condition is assessed for safety and efficacy of the treatment using conventional systems to check their eligibility for stem cell treatment for Brian disorders including Stroke, Axonal Injury, Hypoxic Injury, etc.
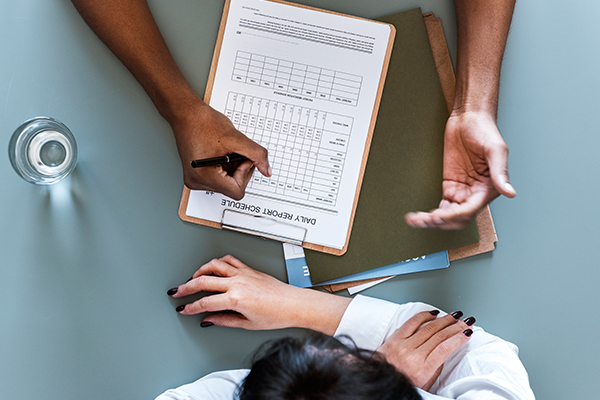
We also conduct an additional round of thorough assessment using our patented NFS (Nutech Functional System) protocols whereby condition-based sets of parameters have been pre-devised for a finer assessment of the patient’s condition (and progress).
After evaluating the specific patient’s condition, a unique/bespoke treatment protocol is decided. This protocol is based on our proprietary stem cell platform, Reostem®.

The patient’s progress is monitored on a weekly basis to further optimise treatment protocols depending on the patient’s response.

At the end of the treatment, a complete assessment is done to track progress from when the patient came into where he/she has reached now. If required, a follow up protocol is recommended.

At Nutech MediWorld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Brain Disorder patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient.

At Nutech MediWorld, we follow an evidence-based, non-invasive, multi-disciplinary approach using Stem Cell Treatment & supporting therapies to improve the condition and overall quality of life for Brain Disorders Patients. Each treatment protocol comprises of the use of our patented technology, customized to suit the condition of the individual patient

At Nutech MediWorld, the patients’ clinical condition is assessed for safety and efficacy of the treatment using conventional systems to check their eligibility for stem cell treatment for Brian disorders including Stroke, Axonal Injury, Hypoxic Injury, etc.

We also conduct an additional round of thorough assessment using our patented NFS (Nutech Functional System) protocols whereby condition-based sets of parameters have been pre-devised for a finer assessment of the patient’s condition (and progress).
After evaluating the specific patient’s condition, a unique/bespoke treatment protocol is decided. This protocol is based on our proprietary stem cell platform, Reostem ®.

The patient’s progress is monitored on a weekly basis to further optimize treatment protocols depending on the patient’s response.

At the end of the treatment, a complete assessment is done to track progress from when the patient came into where he/she has reached now. If required, a follow-up protocol is recommended.
We use SCT-N® and SCT-NX® products, based on a proprietary stem cell platform, ReoStem®. ReoStem® is a unique, replicable, clinical-stage technology platform for the generation of allogenic stem cells.
Nutech MediWorld’s regenerative medicine products are universally applicable: they do not require tissue-matching or the co-administration of immunosuppressive drugs.
There is absolutely no evidence of teratoma generation or tumorigenesis associated with SCT-N® or SCT-NX® use in over 17 years of clinical practice across thousands of patients.
Unique cell culture methodology allows scalability of cell expansion and production.